The "Biological Reset" Consultation
Request Your On-Site Assessment
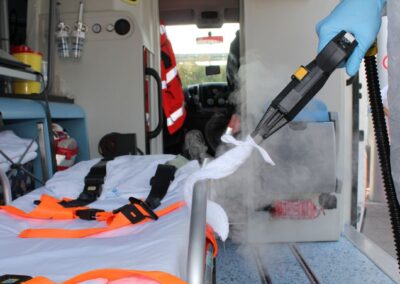
Are you ready to see how Premier Steam can transform your environment with our clinically validated, 300° Thermal Decontamination?
Our Saturated Dry Vapor technology neutralizes the most resilient Pathogen Loads with zero downtime and zero chemical residue. Submit the form below to schedule a professional walkthrough and receive a customized Biological Reset protocol for your facility or home.
The Protocol Advantage:
-
Total Pathogen Neutralization
-
100% Chemical-Free Intervention
-
Forensic Biofilm Eradication
*By submitting this form, you agree to receive communications via text message or phone call regarding your demo appointment. We value your privacy and will only contact you about your appointment. You can opt-out at any time.
Secure Your Biological Reset Protocol
Commercial
Bio-Sanitization & Pathogen Control
Redefining the standard of clinical purity for high-traffic environments. We deploy 300°F Saturated Dry Vapor to ensure the biological integrity of your facility, protecting both your workforce and your professional reputation.

Commercial Bio-Sanitization standards
In an era of heightened environmental awareness, maintaining Biological Integrity within commercial spaces is a critical pillar of public safety and operational success. Our comprehensive Thermal Decontamination service delivers deep Bio-Sanitization, effective pathogen neutralization, and total atmospheric deodorization across various industries. By establishing a clinically validated environment, we protect the health of your stakeholders, enhance consumer confidence, and uphold the uncompromising standards of your professional reputation.

Health & Wellness Facilities
Our Thermal Decontamination protocol ensures an Aseptic Environment essential for high-end spas, holistic clinics, and wellness centers. This 300°F chemical-free intervention maintains a pure, irritant-free atmosphere while achieving total Pathogen Neutralization on all sensitive surfaces, ensuring your sanctuary remains biologically pristine.

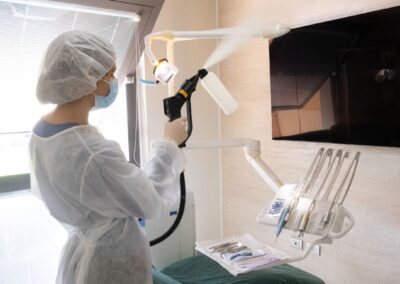
Medical & Professional Suites
In clinical settings, surface integrity is non-negotiable. Our Bio-Sanitization specialists provide a deep Biological Reset for medical offices and corporate suites without leaving corrosive residues or harmful off-gassing. We effectively dismantle Microbial Biofilms, ensuring a safe, professionally validated space for both practitioners and patients.

Food Industry & Culinary Spaces
From commercial kitchens to mobile food units, we specialize in Organic Load Reduction. Our high-velocity dry vapor emulsifies stubborn grease and food-borne pathogens at the source, ensuring a hygienic production environment that exceeds strict health regulations. This Eco-Technical approach guarantees no chemical traces ever interfere with your culinary standards.

Athletic & Physical Fitness Centers
High-traffic fitness environments face constant Pathogen Loading and odor-causing bacteria. Our Thermal Protocols deeply penetrate equipment surfaces and porous floorings to neutralize viral threats and eliminate biological odors at their origin. This process establishes a fresh, high-performance atmosphere that prioritizes member health and asset longevity.
Frequently Asked Questions
How does a "Bio-Reset" differ from standard commercial cleaning?
Traditional janitorial services focus on “surface aesthetics”—removing visible dirt with mops and chemicals. Premier Steam performs a Biological Reset. We utilize Thermostar AISI 304 Stainless Steel technology to deliver 300°F+ Saturated Micro-Dry Vapor at 9 bar of pressure. This doesn’t just move contaminants; it achieves Thermal Lysis, physically destroying the cellular structure of pathogens and biofilms that chemical wipes leave behind.
Do you use harsh chemicals that might violate OSHA or health standards?
No. Premier Steam provides a 100% Chemical-Free Protocol. We rely on pure thermal energy to reach the “Kill Point” of viruses, bacteria, and allergens. This ensures your facility remains free of VOCs (Volatile Organic Compounds) and chemical residues, making it the safest option for healthcare facilities, gyms, and food-service environments.
Does this process affect "down-time" for our business?
Minimal to none. Our Dual-Chamber Thermostar technology allows for continuous operation, and the Micro-Dry Vapor ensures surfaces are dry and ready for use within minutes. We specialize in “After-Hours Bio-Purges” to ensure your facility is decontaminated and ready for operation by the next business morning.
Is your process safe for high-tech medical or office equipment?
Yes. Because our system produces Saturated Micro-Dry Vapor (containing only 5% moisture), it is safe for most electronic interfaces, medical upholstery, and sensitive office environments when handled by our Certified Thermal Technicians. The “dry” nature of the vapor allows for immediate evaporation, meaning there is zero risk of water damage or mold growth—a common failure of traditional steam cleaners.
How do you verify that a surface is actually "sanitized"?
We don’t ask you to take our word for it. We utilize Clinical ATP Luminometer Testing. By swabbing surfaces before and after our protocol, we provide measurable data in RLUs (Relative Light Units). Every commercial client receives a Sanitization Audit Report documenting the bio-load reduction, providing you with a “paper trail” of safety and compliance.
What is the "Thermal Death Point," and why does 300°F matter?
Most pathogens, including MRSA, E. coli, and Flu viruses, have a thermal death point between 140°F and 165°F. However, they hide within Biofilms—protective “slime” layers that act as heat shields. Our 300°F vapor provides the “thermal shock” necessary to blast through the biofilm and ensure the pathogen underneath reaches its death point instantly.

Clinical-Grade Bio-Sanitization & Pathogen Control in Elizabethtown, KY
Bio-Sanitization Service Areas:
Premier Steam proudly serves the Greater Elizabethtown area, including Radcliff, Glendale, Hodgenville, and Fort Knox. We also provide clinical-grade bio-sanitization protocols for partners in Louisville and Bowling Green, KY.
QR Code Share
With the click of a button get us added to your home screen to receive notifications.

Request Your On-Site Precision Quote
Please fill in the form below and we will be in touch with you as soon as possible.